Why perovskite solar cells often tend to set apart drunk of light
- Solar cells made of perovskite are cheap, very easy to create, and also almost as efficient as silicon, the product generally utilized in solar cells.
Nonetheless, perovskite cells have a love-hate-relationship with the Sun. The light that they require to create electrical power additionally occurs to harm the high quality of the cells, thus significantly limiting their effectiveness and security over time. Scientists at Eindhoven University of Modern technology have currently developed a concept that explains why compound perovskite cells are unpredictable in sunshine. The research study was published in Nature Communication
Perovskite is an eye-catching choice to silicon for solar cells since it's bountiful and also simple to produce. What's more, over the past decade, the performance of perovskite solar cells has boosted considerably, with performance rates getting to more than 25%, near the state-of-art for silicon solar cells.
Perovskite photovoltaic panels are normally made from halide compounds, which incorporate natural products such as methylammonium or formamidinium with a steel (typically lead or tin) as well as halides like bromide or iodide. The new research concentrates on five prominent halide compounds, which have in common that they combine both bromide and iodide.
This mix functions specifically well since it enables the 'tuning' of the band space, or the minimum amount of photon energy required to produce electrical energy in the material. This is especially handy in so-called tandem solar cells where perovskite is combined with silicon and which call for excellent 'adjusting' of the various band spaces to make certain optimal performance.
Solving the enigma of segregation
However, there is a snag. While sunlight provides the perovskite cells with the photon energy they need to produce electrical power, it likewise impairs their security. With time, this affects their performance. While this love-hate partnership in between perovskites and the sun is well-known in the field, it is hardly understood.
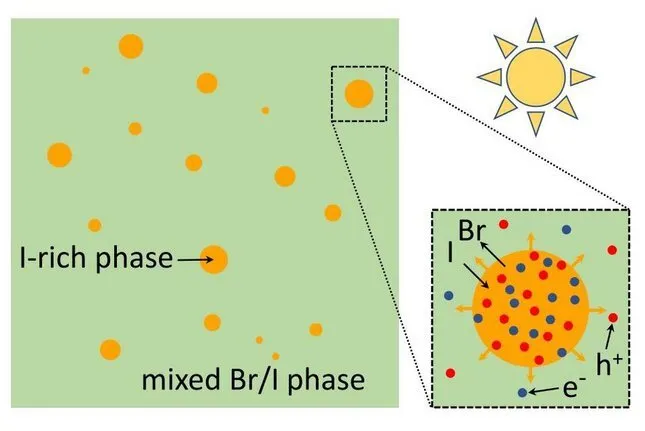
" To see what is taking place, you need to take a look at what is occurring at the atomic level when light goes into a solar cell," says Peter Bobbert, professor at the division of Applied Physics and also co-author of the article. "The photocarriers (electrons as well as openings), which are created by the inbound photons, have a tendency to collect at areas where the band space is lowest, in this case the iodide-rich regions that spontaneously occur in the substance."
Given there suffices light, this triggers a domino effect in which a growing number of iodide builds up in the iodide-rich regions, and also more and more bromide is eliminated. The succeeding segregation of the substance often tends to catch the electricity-producing photocarriers in these low-band gap locations, significantly hampering the effectiveness of the cell.
Free energy
The researchers-- who collaborate in the Facility for Computational Power Research Study and besides Peter Bobbert include Zehua Chen, Geert Brocks and also Shuxia Tao-- have created an academic model that can clarify why this is taking place. "Our combined theory for light-induced halide segregation looks at the overall totally free power of the perovskite in the photovoltaic cells, both in the dark and when the cell is subjected to sunshine," discusses Bobbert.
" Excited photocarriers often tend to relocate to locations where their cost-free energy is cheapest, in this situation the locations with the lowest band space. By evaluating this procedure, we were able to distinguish between steady, metastable, and also unpredictable regions for each compound, relying on the temperature, the light problems, and the bromide concentration."
Solutions
The gorgeous thing about the theory is that it discloses a sensible path in the direction of possible solutions for the issue of substance segregation. One of the most obvious solution is restricting the light that falls on the cell, yet that would detrimentally impact efficiency. An even more appealing means is to change the quantity of bromide in the compound.
" Based upon our calculations, you can forecast exactly how much bromide you can include in the compound to enhance its band gap without making the compound unpredictable," claims Bobbert. "By not mixing in an excessive quantity of bromide, you can prevent segregation, while at the same time still attaining a fairly huge band gap that works for tandem cells."
The paper offers the instance of a cesium-lead substance cell that is secure up to 42% bromide focus (under typical temperature and also sunshine problems). This ought to ensure an optimum band void of 1.94 eV, greater than enough for the top layer in an effective tandem solar cell.
Other services
According to the researchers, the theory is extremely flexible, which makes it applicable to other semiconductor materials where the band void is tuned via alloying with additional materials as well as to situations that have actually not yet been considered, such as modifications in the band void due to strain.
Also read

