Scientist gain better understanding of the stability origin of Dion-Jacobson 2D perovskites
- A research study group, led by Prof. GUO Xin and Prof. LI Can from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), has actually unveiled the origin of the stability of Dion-- Jacobson (DJ) phase two-dimensional (2D) perovskite materials.
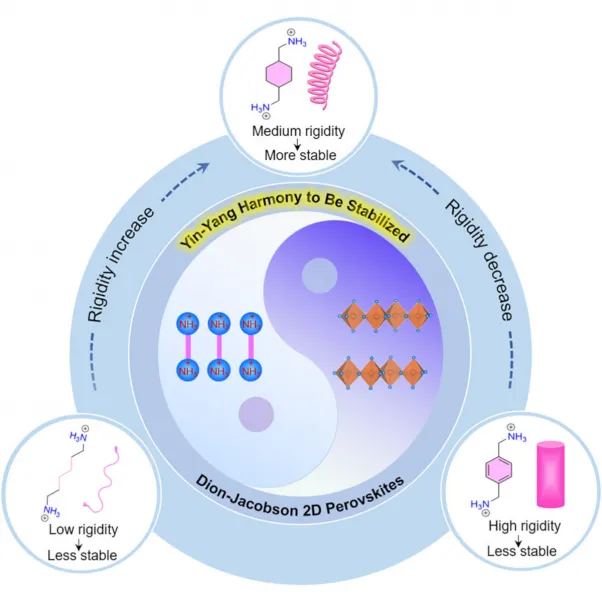
Compared to traditional 3D perovskites with inherently low stability, 2D perovskites including the Ruddlesden-- Popper (RP) phase as well as DJ phase are thought about appealing as a result of their boosted stability, especially DJ 2D perovskites based on organic diammonium cations and also inorganic lead iodide octahedra, which have higher structural stability than RP ones. Nevertheless, some of DJ 2D perovskites are not durable as well as break down even more conveniently than their RP 2D counterparts, which has actually brought about a dispute over their stability. These inconsistent research study results have impelled scientists to search for much better understanding regarding the stability device of DJ 2D perovskites with various diammonium cations.
In their research study, the researchers showed that the stability of DJ 2D perovskites was determined by the strength of organic diammonium cations, which might cause co-adaptation of organic as well as inorganic components to stabilize the 2D structure. Specifically, the medium cation rigidness permitted organic diammoniums and not natural octahedra to regulate their geometries in mutual holiday accommodation in order to reach a stabilized state.
This system might supply a path for scientists who want to knowingly control the stability of DJ 2D perovskites by changing cation strength.
" Comparable to both events in the Yin-Yang concept, organic cations and inorganic octahedra in DJ 2D perovskites are adjoined with alternating hydrogen bonds as well as when both components can readjust their geometries to adjust to each various other, solid connections are preferable," claimed Prof. GUO. "By doing this, organic cations with moderate rigidness would certainly play a crucial duty in the process of intercoordination since they have a certain degree of liberty to manage the geometry as well as the not natural component can correspondingly alter its arrangement too."
Also read

