Princeton chemists resolve beginning of perovskite instability
- Scientists in the Cava Group at the Princeton University Department of Chemistry have demystified the reasons for instability in an inorganic perovskite that has actually brought in large attention for its potential in creating extremely reliable solar cells.
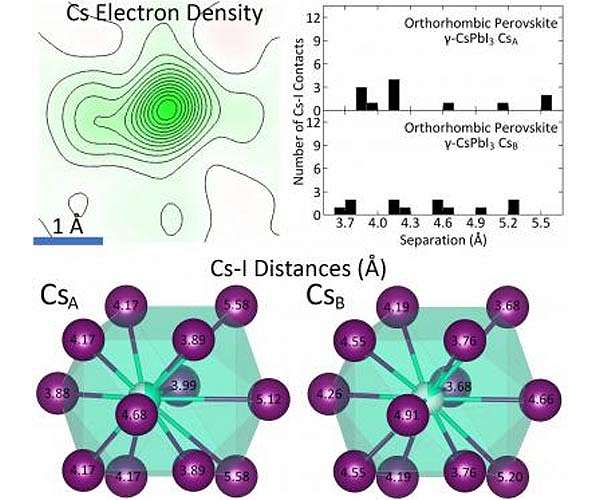
Utilizing single crystal X-ray diffraction done at Princeton University as well as X-ray set circulation function dimensions performed at the Brookhaven National Laboratory, Princeton Department of Chemistry researchers found that the source of thermodynamic instability in the halide perovskite cesium lead iodide (CsPbI3) is the inorganic cesium atom as well as its "rattling" actions within the crystal structure.
X-ray diffraction generates a clear experimental trademark of this motion.
The research, "Understanding the Instability of the Halide Perovskite CsPbI3 via Temperature-Dependent Structural Analysis," will be published next week in the journal Advanced Materials.
Daniel Straus, a postdoctoral research affiliate in the Cava Group and also lead writer on the paper, clarified that while cesium inhabits a solitary site within the framework at temperatures listed below 150 K, it "splits" right into two sites above 175 K. Along with various other structural criteria, this recommends evidence of the rattling actions of cesium within its iodine coordination polyhedron.
Additionally, the reduced variety of cesium-iodine get in touches with within the framework as well as the high degree of neighborhood octahedral distortion likewise add to the instability.
In the study, the single-crystal dimensions characterized the average framework of the product. At Brookhaven, the X-ray pair distribution feature allowed scientists to figure out the behavior of the structure on the size range of the system cell. (A system cell is the tiniest repeating device in a crystal.) It gets on this neighborhood level that the high degree of octahedral distortion ended up being evident, stated Straus.
The room-temperature metastability of CsPbI3 has long been a recognized factor, however it had not previously been discussed.
" Finding a description for an issue that so many individuals in the research study community want is fantastic, as well as our partnership with Brookhaven has been beyond fantastic," claimed Robert Cava, the Russell Wellman Moore Professor of Chemistry, a specialist in synthesis and structure-property characterization.
" Remarkable" efficiencies
Presently, the leading halide perovskite in solar energy conversion applications is based on methylammonium lead iodide, an organic-inorganic hybrid material that has been incorporated into solar cells with certified efficiencies of 25.2%; this equals the effectiveness of industrial silicon solar cells. While this "exceptional" effectiveness drives rate of interest, methylammonium lead iodide struggles with instability issues believed to originate from the unstable nature of the natural cation. To fix this problem, researchers have tried to replace the organic cation with not natural cesium, which is significantly much less volatile.
However, unlike methylammonium lead iodide, the perovskite stage of cesium lead iodide is metastable at area temperature.
" If you want to make a solar cell with unmodified cesium lead iodide, it's mosting likely to be extremely tough to function about this and support this material," stated Straus. "You have to find a way to maintain it that functions around the truth that this cesium atom is a little too tiny. There are a pair ways individuals have attempted to chemically change CsPbI3 and also they work fine. However there's no factor in just attempting to make solar cells out of this bulk material without doing fancy things to it."
In-depth structural information in the paper recommends techniques to support the perovskite stage of CsPbI3 as well as hence boost the stability of halide perovskite solar cells. The paper also discloses the limitations of tolerance factor models in forecasting security for halide perovskites. Most of these versions presently anticipate that CsPbI3 should be secure.
At Brookhaven Lab
A technique referred to as a set circulation function measurement, which explains the circulation of ranges in between atoms, aided the Princeton scientists to additionally comprehend the instability. Using Brookhaven's Pair Distribution Function (PDF) beamline at the National Synchrotron Light Source II, lead beamline researcher Milinda Abeykoon worked with examples of thermodynamically unsteady CsPbI3, which he received from the Cava Lab in numerous covered glass veins inside a container loaded with solidified carbon dioxide.
Measuring these examples was difficult, stated Abeykoon, since they would certainly decompose rapidly when removed from the dry ice.
" Thanks to the exceptionally brilliant X-ray light beam and large location detectors offered at the PDF beamline, I had the ability to measure the samples at numerous temperatures below 300 K before they deteriorated," stated Abeykoon. "When the X-ray beam of light bounces off the sample, it creates a pattern feature of the atomic arrangement of the material. This offers us the opportunity to see not just what is taking place at the atomic scale, yet additionally just how the product acts as a whole in one dimension."
Cava lauded the 45-year relationship he has actually had with Brookhaven, which started with experiments he completed there for his Ph.D. thesis in the 1970s. "We have had several wonderful cooperations with Brookhaven," he stated.
