Mystery of amorphous perovskite resolved
- AMOLF researchers Erik Garnett, Susan Rigter, and also colleagues are the initial to have irrefutably shown that amorphous perovskite exists. The material can dramatically increase the efficiency of solar cells produced from perovskite. The research study is published today in the journal Advanced Functional Products.
Perovskite, the very appealing new product for solar cells, is naturally crystalline; to put it simply, the atoms pack together in an ordered pattern. From typical silicon solar cells, we know that the effectiveness of the cells can be boosted if a part of the material is amorphous, meaning the atoms pack together randomly.
Erik Garnett (AMOLF Nanoscale Solar Cells) was the first to understand that amorphous perovskite might accomplish the very same function. The adhering to difficulty was to create the product as well as examine its properties. Garnett clarifies why that was challenging: "Perovskite includes ions. Naturally, these quickly organize in a crystal lattice, much like salt, for instance. We needed ahead up with a technique to avoid those crystals from developing, as well as we managed to do just that. Using methods such as X-ray diffraction, we consequently additionally demonstrated that the product is amorphous. With this, we provided the very first irrefutable proof that amorphous perovskite exists."
Vinegar makes perovskite amorphous
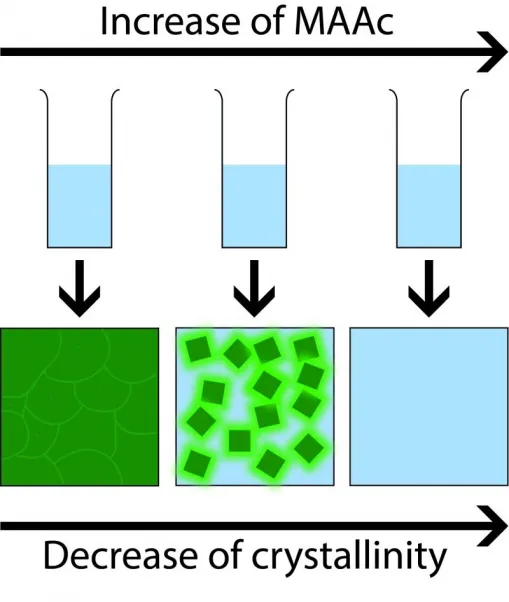
The method that Garnett, very first writer of the short article Susan Rigter, as well as their colleagues applied is varying the amount of methylammonium acetate, one of the components of perovskite. Extra acetate (the key active ingredient in vinegar) leads to even more amorphous perovskite since it impedes the formation process and speeds up the disappearance of the solvent. "We were actually shocked that we can form amorphous perovskite, so we wished to explore the formation system," states Garnett. "We demonstrated that as an intermediate phase, a facility is developed in the remedy that impedes formation. When we ultimately heat up the solution to evaporate the solvent, the complicated disintegrates so rapidly that it does not have time to take shape."
The technique that the scientists devised to make amorphous perovskite is widely relevant. One of the most examined perovskite is methylammonium lead iodide, yet the synthesis likewise deals with other ammonium salts as well as with various other halides such as bromide rather than iodide. In addition, it transpired that varying these elements generated a change in the bandgap, a residential or commercial property of the substance that shows which color light the solar cell takes in and also exchanges electrical energy most effectively. The capacity to tune the amorphous band space allows several materials with different bandgaps to be incorporated, bring about more effective solar cells
Reliable solar cells.
Comparable to silicon solar cells, an amorphous layer of perovskite can aid boost the efficiency by supplying a supposed passivating layer, says Garnett. Electrons are released in the product as a result of light beaming on a solar cell. These electrons move to the surface area where they are eliminated through digital get in touches with. This gives rise to a current. In a crystal, the electrons can end up being entraped at the border of the crystal. In document silicon solar cells, an amorphous passivating layer makes certain that this does not take place, leading to higher power result from the solar cell. Amorphous perovskite could additionally accomplish this function, which would further increase the performance of perovskite solar cells. "We determine stronger and longer lived light exhaust when making use of the amorphous perovskite as a passivating layer, which is an indication for a far better doing solar cell," claims Garnett.
Consequently, the following step in the research study is generating this type of solar cell, beginning with a layer of crystalline perovskite that is covered by a layer of amorphous perovskite. That is harder than creating just amorphous perovskite because the underlying crystalline layer gives a bought layout, making it less complicated for atoms to pack in a bought style. "I consider the example with silicon to be one of the most interesting facet of our research," says Garnett. "I think that this is a considerable breakthrough for perovskites with significant possibilities."
Also read

