Controlling perovskite ions' composition paves the way for tool applications
- Hybrid organic-inorganic perovskites (* 1) have obtained much attention as possible future generation solar cells and also as materials for light-emitting devices.
Kobe University's Associate Professor TACHIKAWA Takashi (of the Molecular Photoscience Research Center) as well as Dr. KARIMATA Izuru (previously a graduate student participated in research study at the Graduate School of Science) have succeeded in entirely substituting the halide ions of perovskite nanocrystals while maintaining their morphology and also light-emitting performance.
Additionally, by utilizing techniques such as single-particle photoluminescence imaging, the scientists were able to comprehend the momentary modifications in light discharge and also the crystal framework, which subsequently enabled them to establish a principle for managing ion structure.
It is expected that these research outcomes will contribute towards allowing the synthesis of perovskites of differing make-ups as well as progressing the development of tools which use them. Additionally, it is hoped that the flexibility of perovskite frameworks can be harnessed, enabling them to be put on gadgets as well as the production of brand-new practical products.
These searchings for were released in the German academic journal 'Angewandte Chemie International Edition' on October 19, 2020.
Research study Background
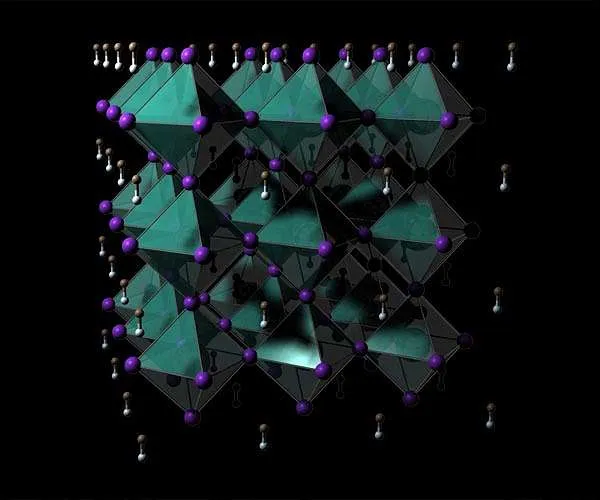
Hybrid organic-inorganic perovskites, such as natural lead halide perovskites (as an example, CH3NH3PbX3 (X = Cl, Br, I)), have actually been getting globally interest as an appealing material for extremely reliable solar cells (Figure 1). Moreover, the shade of the light that they give off can be managed by modifying the type and also structure of the halide ions. As a result, it is wished that hybrid organic-inorganic perovskites can be applied to light-emitting tools such as screens as well as lasers.
Nonetheless, the halide ions inside the crystals are understood to walk around also at space temperature, and this high flexibility triggers issues such as decreases in both synthesis reproducibility and also tool resilience.
Research study Methodology
In this research, the researchers made use of a custom-made flow reactor (* 3) to exactly manage the exchange reaction in between the CH3NH3PbI3 nanocrystals as well as Br- ions in solution. This allowed them to effectively transform the nanocrystals into CH3NH3PbBr3 nanocrystals while maintaining their morphology and also light-emitting effectiveness (Figure 2).
It is essential to understand what kind of reaction will occur inside the crystals in order to develop synthesis techniques. To comprehend this, the scientists made use of a fluorescence microscopic lense to observe just how each private nanocrystal was responding.
From this observation, they understood that as soon as the red light released by the CH3NH3PbI3 had actually entirely disappeared, the green light stemming from the CH3NH3PbBr3 was unexpectedly produced after an interval of 10s to 100s of secs (upper part of Figure 2).
Based upon the outcomes of structural analysis using an x-ray light beam, it was disclosed that Br- ions replaced I- ions inside the crystal framework while a bromide-rich layer based on the surface. Afterwards, the bromide externally layer progressively moved right into the internal regions.
It is believed that the red light discharges ended up being unobservable due to the fact that the internal regions of the crystal framework were partly disordered throughout the ion change, which led to the loss of energy essential for light emission (base of Figure 2). Ultimately, CH3NH3PbBr3 crystal nuclei created inside the nanocrystal particle and a participating transition to the green light generating state occurred.
From these results, it can be claimed that temporally separating the crystal structure shifts as well as the subsequent restructuring (that takes place on a nanometer scale) is one of the secrets to the successful, accurate synthesis of organic lead halide perovskites.
Additional Developments
The architectural change procedure observed in perovskite nanocrystals in this study is believed to be related to all modes of nanomaterial synthesis that are based upon ion exchange, consequently future research study might ideally light up the underlying mechanism.
Although scientists have an adverse impression of natural halide perovskites' flexibility, it is really hoped that this particular could be made use of as well as put on the advancement of brand-new products and devices that can respond to the environment and also exterior stimuli.
Also read

