Unraveling the interfacial interactions of lead-free perovskite for efficient hydrogen manufacturing
- The conversion of solar power into hydrogen energy stands for a promising and also eco-friendly technique for resolving the energy shortage and also reducing fossil fuel discharges. A research group from City University of Hong Kong (CityU) lately established a lead-free perovskite photocatalyst that delivers very reliable solar energy-to-hydrogen conversion.
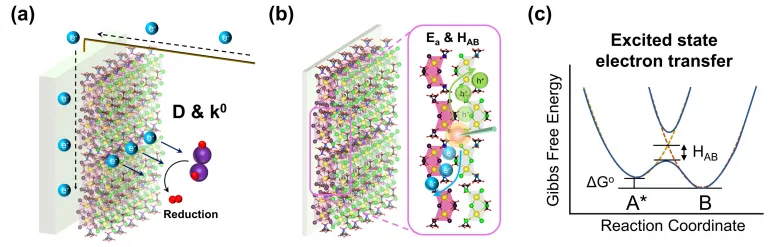
Most significantly, they revealed the interfacial dynamics of solid-solid (in between halide perovskite molecules) and also solid-liquid (between a halide perovskite as well as an electrolyte) interfaces during photoelectrochemical hydrogen manufacturing. The latest findings open a method to create a much more effective solar-driven method for producing hydrogen fuel in the future.
Hydrogen is thought about to be a much better as well as more encouraging renewable resource option as a result of its abundance, high energy density, and also ecological friendliness. Besides photoelectrochemical water splitting, one more encouraging method of generating hydrogen is by splitting hydrohalic acid using solar-driven photocatalysts. Yet the lasting stability of photocatalysts is a vital challenge, as a lot of catalysts made from shift metal oxides or metal are unsteady under acidic problems.
" Lead-based hybrid perovskites are made use of to conquer this stability concern, but the high solubility in water as well as toxicity of lead limits their capacity for widespread applications," clarified Dr. Sam Hsu Hsien-Yi, Assistant Professor in the School of Energy and Environment and the Department of Materials Science as well as Engineering at CityU.
" Bismuth-based perovskites, on the other hand, have been confirmed to offer a non-toxic, chemically stable alternative for solar-fuel applications, but the photocatalytic efficiency requires to be improved."
Motivated to create an efficient and stable photocatalyst, Dr. Hsu and also his partners recently established a bismuth-based halide perovskite with a structure of bandgap funneling for highly reliable charge-carrier transportation. It is a mixed-halide perovskite, in which the circulation of iodide ions gradually lowers from the surface to the interior, forming a bandgap funnel framework, which advertises a photo-induced fee transfer from the interior to the surface for an efficient photocatalytic redox reaction.
This recently designed perovskite has high solar energy conversion performance, exhibiting a hydrogen generation rate enhanced approximately about 341 ± 61.7 µmol h − 1 with a platinum co-catalyst under noticeable light irradiation. The findings were published in Small Methods.
But Dr. Hsu's group did not stop there. "We wished to explore the dynamic interactions between the halide perovskite molecules as well as those at the interface between the photoelectrode and the electrolyte, which remained unknown," said Dr. Hsu.
" Given that photoelectrochemical hydrogen production involves a catalytic procedure, highly effective hydrogen generation can be accomplished by intense light absorption utilizing a semiconductor as a photocatalyst with an ideal energy band framework and also efficient cost separation, helped with by an exterior electric area formed near the semiconductor-liquid interface."
To uncover the exciton transfer dynamics, the group utilized temperature-dependent time-resolved photoluminescence to analyze the energy transport of electron-hole pairs between the perovskite molecules. They likewise assessed the diffusion coefficient as well as electron transfer rate constant of halide perovskite materials in the solution to illustrate the efficiency of electron transportation via the solid-liquid interfaces between a perovskite-based photoelectrode and the electrolyte.
" We demonstrated how our freshly made photocatalyst can effectively attain high-performance photoelectrochemical hydrogen generation as a result of efficient cost transfer," stated Dr. Hsu.
In the experiment, the team additionally proved that bandgap funneling structured halide perovskites had an extra effective cost separation as well as transfer procedure between the interface of the electrode as well as electrolyte.
The boosted fee separation can drive the migration of cost providers onto the surface of halide perovskites transferred on the conductive glasses as the photoelectrode, allowing much faster photoelectrochemical activity on the photoelectrode's surface. Consequently, the effective charge transfer inside the bandgap funnel organized halide perovskites exhibited improved photocurrent density under light irradiation.
" Uncovering the interfacial dynamics of these novel materials throughout the procedure of photoelectrochemical hydrogen generation is a critical advancement," discussed Dr. Hsu. "A comprehensive understanding of the interfacial interactions between halide perovskites and liquid electrolytes can construct a scientific structure for researchers in this area to additionally investigate the advancement of choice and also beneficial materials for solar-induced hydrogen manufacturing."
The searchings for were published in Advanced Materials.
Also read

