Twisting, flexible crystals essential to solar power manufacturing
- Scientists at Duke University have actually revealed long-hidden molecular characteristics that supply preferable properties for solar energy and heat energy applications to an interesting class of products called halide perovskites.
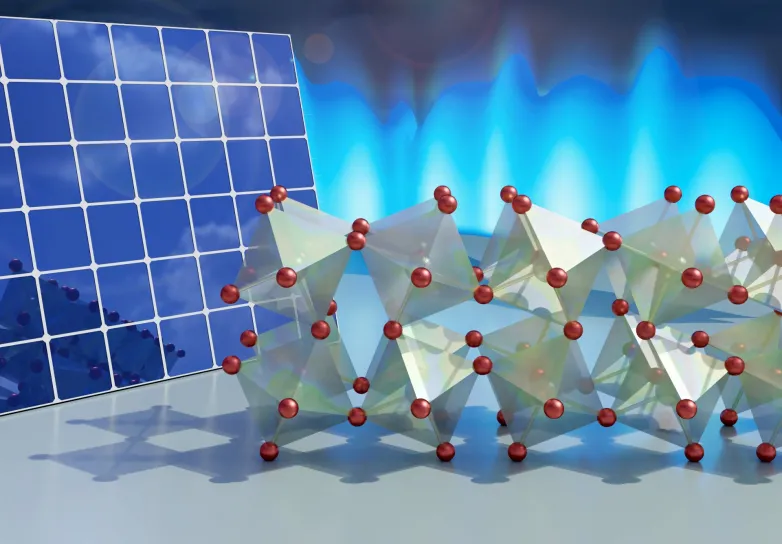
An essential contributor to how these products develop as well as transfer electricity literally rests on the means their atomic lattice weaves in a hinge-like style. The outcomes will aid materials scientists in their pursuit to tailor the chemical recipes of these products for a wide variety of applications in an eco-friendly method.
The results show up on-line March 15 in the journal Nature Materials.
" There is a broad rate of interest in halide perovskites for power applications like photovoltaics, thermoelectrics, optoelectronic radiation discovery and also discharge-- the entire area is extremely energetic," claimed Olivier Delaire, associate teacher of mechanical engineering as well as materials scientific research at Duke. "While we understand that the soft qualities of these products is very important to their digital properties, no one really recognized just how the atomic activities we have actually uncovered underpin these attributes."
Perovskites are a course of materials that-- with the ideal combination of aspects-- are grown into a crystalline framework that makes them especially fit for energy applications. Their ability to take in light as well as transfer its power successfully makes them a typical target for scientists creating brand-new kinds of solar batteries, for example. They're likewise soft, sort of like how solid gold can be conveniently dented, which provides the capability to endure flaws and stay clear of splitting when made into a thin film.
One dimension, nevertheless, does not fit all, as there is a vast array of potential dishes that can form a perovskite. A lot of the most basic and most studied recipes include a halogen-- such as chlorine, fluorine or bromine-- giving them the name halide perovskites. In the crystalline structure of perovskites, these halides are the joints that tether adjoining octahedral crystal motifs with each other.
While researchers have known these pivot factors are vital to developing a perovskite's residential or commercial properties, no one has actually had the ability to look at the method they permit the structures around them to dynamically twist, turn and bend without breaking, like a Jell-O mold being strongly drunk.
" These architectural movements are infamously hard to pin down experimentally. The method of option is neutron spreading, which comes with enormous instrument as well as data evaluation effort, and extremely couple of groups have the command over the technique that Olivier and his associates do," said Volker Blum, professor of mechanical design as well as product science at Duke that does academic modeling of perovskites, but was not involved with this research study. "This implies that they are in a setting to expose the foundations of the materials homes in standard perovskites that are otherwise inaccessible."
In the study, Delaire and also colleagues from Argonne National Laboratory, Oak Ridge National Laboratory, the National Institute of Standards and Technology, and also Northwestern University, expose important molecular characteristics of the structurally straightforward, typically looked into halide perovskite (CsPbBr3) for the very first time.
The researchers started with a huge, centimeter-scale, solitary crystal of the halide perovskite, which is notoriously difficult to expand to such sizes-- a major reason this kind of vibrant research has not been achieved before now. They after that barraged the crystal with neutrons at Oak Ridge National Laboratory and also X-rays at Argonne National Laboratory. By measuring how the neutrons and also X-rays bounced off the crystals over numerous angles as well as at different time intervals, the researchers teased out exactly how its component atoms conformed time.
After confirming their interpretation of the dimensions with computer system simulations, the researchers discovered simply how active the crystalline network really is. Eight-sided octahedral concepts affixed to one another through bromine atoms were captured twisting jointly in plate-like domains and also frequently bending backward and forward in a very fluid-like fashion.
" Due to the means the atoms are organized with octahedral motifs sharing bromine atoms as joints, they're cost-free to have these turnings and bends," said Delaire. "But we discovered that these halide perovskites in particular are much more 'floppy' than a few other recipes. As opposed to quickly bouncing back into form, they return very gradually, almost more like Jell-O or a fluid than a standard strong crystal."
Delaire explained that this free-spirited molecular dancing is important to recognize much of the desirable buildings of halide perovskites. Their 'floppiness' quits electrons from recombining into the holes the incoming photons knocked them out of, which helps them make a lot of electrical energy from sunshine. And also it likely additionally makes it challenging for heat to travel throughout the crystalline structure, which allows them to create electrical energy from heat by having one side of the product be much hotter than the various other.
Since the perovskite utilized in the research study-- CsPbBr3-- has one of the easiest recipes, yet already contains the architectural attributes typical to the wide family of these compounds, Delaire thinks that these searchings for likely apply to a big series of halide perovskites. As an example, he cites hybrid organic-inorganic perovskites (HOIPs), which have a lot more complex dishes, as well as lead-free double-perovskite variants that are extra eco-friendly.
" This research reveals why this perovskite structure is special even in the simplest of cases," said Delaire. "These findings highly likely include much more complex recipes, which lots of scientists throughout the world are presently investigating. As they evaluate massive computational data sources, the characteristics we have actually revealed could help choose which perovskites to pursue."
Also read

