Solar cells motivate Li-ion batteries
- Solar power goes to the center of the international shift toward generating sustainable power resources as well as dealing with power poverty.
Nonetheless, the periodic nature of solar energy limits its usage for applications such as IoT gadgets, live remote sensing and also an off-grid power supply. Conventionally the batteries are powered by solar cells to keep the energy for later use. Nevertheless, the physical combination of these two modern technologies needs different product packaging of both systems, is cumbersome to install, as well as requires extra electrodes, which boosts the expense and also ohmic losses in the tool.
Additionally, these physically connected photovoltaic (PV) panels and batteries take advantage of different kinds of energy materials to achieve both power harvesting and storage, that makes the overall system bulky. Consequently, these issues limit applications.
In this context, the demonstrated photorechargeable batteries (PRBs) can offer an appealing solution to get rid of the restrictions connected with the physical integration of PVs as well as batteries. A PRB can do solar power harvesting and storage space simultaneously in a solitary gadget making use of advanced nanomaterials, which can perform energy harvesting and storage effectively. This cutting-edge modern technology assures to be lightweight as well as reliable contrasted to the existing conventional mix of PVs and batteries.
Light-induced conversion device for PRBs
In their study released in Advanced Sustainable Solutions, scientists at the Advanced Energy Materials Lab, Division of Physics at the Indian Institute of Innovation Jodhpur have demonstrated that iron oxide (also known as hematite) nanorods can work as an energetic product to develop efficient and low-priced photocathodes for PRB applications. The high academic specific capacity (1006 mAh g-1), planet wealth, nontoxicity, environmental friendliness as well as reduced handling techniques make the alpha stage of iron oxide an eye-catching anode material for lithium-ion batteries.
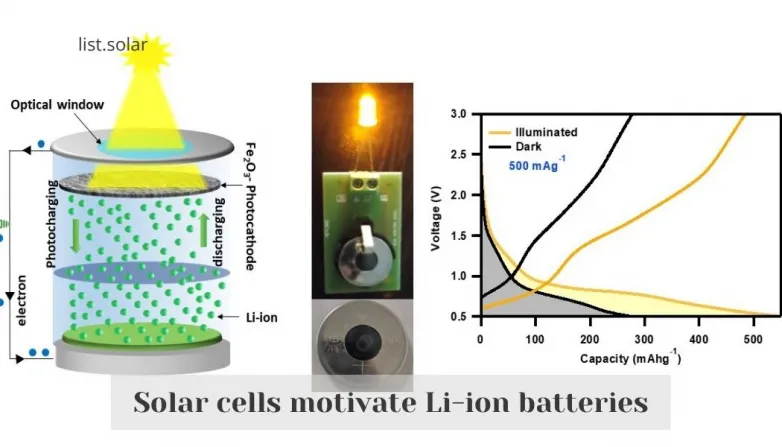
The iron oxide nanorods have revealed the capacity to at the same time gather the solar radiation in the visible area due to their bandgap of ~ 2.1 eV and store the Li-ions effectively. This job provides the first demo of standalone photocharging by exploring the conversion response mechanism where greater than 90% improvement in the specific capacity of the lithium-ion battery is achieved upon solar lighting.
"The very nanoporous photocathodes are produced using hematite, C-61 carbon (PCBM) as well as carbon nanotubes. Hematite can take in sunlight and also produce photogenerated fee service providers, while PCBM and also carbon nanotubes conductive additives gave an ideal path for photogenerated electrons to reach the present enthusiast and also start photocharging," claimed Shubham Chamola, the very first writer of the research post.
The nanoporous iron oxide nanorods are fabricated by using an inexpensive service processing technique in which the carbon nanotubes are functionalized with PCBM and also combined with the NMP service of hematite nanoparticles, which led to the growth of Fe2O3 nanorods on carbon nanotubes packages. To assemble the photorechargeable battery, the scientists used a customized 2032 coin cell casing with an 8 mm diameter opening punched under to act as an optical home window to couple light. This window was secured with a transparent PVC sheet and also the energetic product based photocathode was put encountering towards the window.
The PRB revealed independent billing when lit up with a 470 nm blue LED, accomplishing a photoconversion and storage efficiency (PCSE) of 1.988%, which is a considerable success in the field of PRBs contrasted to the earlier published outcomes based upon the intercalation-based ion storage space. Under white light LED illumination at a high existing price of 2,000 mA g-1, the PRB revealed a 92.96% enhancement in specific capacities. When the PRB is revealed to light, Fe2O3 nanorods soak up photons of power greater than their power band void as well as generate photogenerated fee providers at the photocathode. The conductive ingredients offer a favorable pathway for photoelectrons to get to the current collector and better the anode via an exterior circuit.
Simultaneously, photoholes present in Fe2O3 oxidize Fe0 to Fe3+ which supplies repulsion to the Li+ toward the Li-metal anode using the electrolyte. As a result, the Li-ions are decreased at the anode to form Li-metal, resulting in photocharging. This phenomenon was more shown by videotaping the OCV under a repellent discharge and when the light was activated, the OCV began to enhance-- a phenomenon that contrasts what would normally be anticipated. This recommends that more photogenerated charge service providers are present than are needed to meet the existing demand for releasing the PRB.
This phenomenon could be beneficial where continuous procedure of sensors is called for, making PRBs capable of charging during daytime hours and using the saved energy to power the tools at night or when there is no sunlight. The underlying device was further studied by performing various electrochemical dimensions, such as OCV evaluation under various resistances, electrochemical resistance spectroscopy, as well as cyclic voltammetry under dark and also illuminated conditions. Additionally, the PRB had the ability to power a business 3V LED even after 3 months of fabrication, revealing that the shown iron oxide nanorods-based PRB are very secure and also do not suffer from self-discharge concerns.
Also read

