Scientists discover innovative approach to make novel lithium-ion battery materials
- Scientists at Boise State University have actually developed a new approach to making novel lithium-ion battery materials. Starting from an amorphous (i.e., a material lacking long-range order) niobium oxide, the group found that the very act of cycling the material with lithium induces a transformation to a novel crystalline Nb2O5 anode with phenomenal Li storage space and fast biking. This process can possibly be made use of to make various other lithium-ion battery materials that can not be quickly made via traditional means.
The study, jointly led by scientists in the labs of Hui (Claire) Xiong, a professor of materials science and engineering at Boise State University, and also Shyue Ping Ong, a professor of nanoengineering at the University of California San Diego, was released in Nature Materials.
The exploration of new materials for lithium-ion batteries has actually tackled a renewed necessity. Fueled by increasing gas prices, there has actually been a surge popular for electrical vehicles (EVs), as well as with it, for the lithium-ion batteries that power them. However, today's lithium-ion batteries are still also pricey and also fee as well slowly.
" Lithium-ion batteries are the leading innovation for the rechargeable battery market, however there's also a rise in the demand for the battery to have high energy, and also quicker charging times," stated Pete Barnes, a Ph.D. alumnus of Xiong's Electrochemical Energy Materials Lab in the Micron School of Materials Science and also Engineering and also the lead author of the work. "If you want to charge your EV for 15 minutes and then jump on the roadway for the next 200 or 300 miles, you require new battery electrodes that can be charged at a very fast rate without much loss in performance."
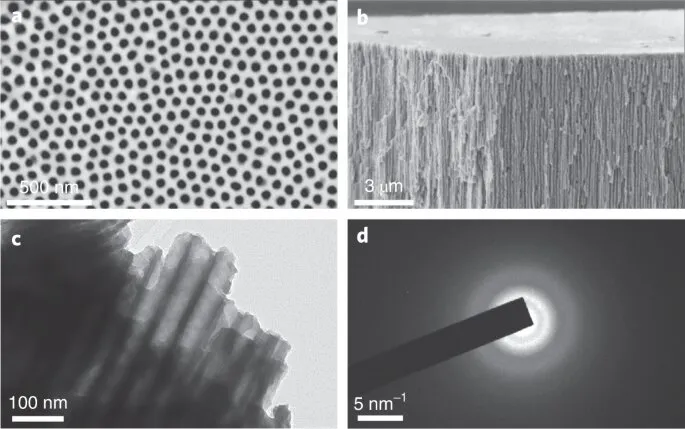
Amongst among the biggest bottlenecks to charging in today's lithium-ion batteries is the anode. The most common anode is made of graphite, which is extremely energy dense, but can not be charged too quickly as a result of the risk of fire and explosions from a procedure referred to as lithium metal plating. Intercalation metal oxides, like the rock salt Nb2O5 material uncovered by the team, are promising anode alternatives as a result of the decreased danger of lithium plating at reduced voltages.
To create the new anode material, Xiong's team created an innovative new technique called electrochemically-induced amorphous-to-crystalline makeover. The new electrode can achieve high lithium storage of 269 mAh/g at a charging rate of 20 mA/g, and much more significantly, remains to preserve a high capacity of 191 mAh/g at a high charging rate of 1 A/g.
" One of the most exciting element of this work is the discovery of a completely new approach to create novel lithium-ion battery electrodes," stated Xiong. "The method is to begin with a greater energy phase, such as an amorphous material. Simply cycling the material with lithium permits us to create new crystalline arrangements that exhibit boosted residential properties beyond those made via traditional means such as solid-state reactions."
The outstanding rate performance of the anode is due to its disordered rock salt or DRX framework, which resembles ordinary kitchen table salt but with the Li as well as Nb atoms arranged in an arbitrary fashion. While DRX cathode materials are widely known, DRX anodes are reasonably rare. Utilizing computational techniques, Yunxing Zuo, a Ph.D. alumnus of Ong's Materials Virtual Lab at UC San Diego, revealed that the process of putting Li into amorphous Nb2O5 permits materials scientists to access metastable materials. The team likewise developed a statistics to identify other metal oxides that can possibly be manufactured in a comparable fashion. The computations also show that the DRX structure consists of paths for fast lithium diffusion, causing high rate performance.
" We believe this work to be simply the start of an entirely new way of thinking about materials synthesis," said Ong. "Atoms like to prepare themselves in specific ways. When we make materials the traditional way, we generally obtain the very same arrangements again and again. This new approach opens up a promising avenue for creating various other unconventional metal oxides."
The team also collaborated with Drs. Sungsik Lee, Justin Connell, Hua Zhou, and Yuzi Liu from Argonne National Laboratory, Profs. Paul Davis, Paul Simmonds, and also Dr. Darin Schwartz from Boise State, and also Drs. Yingge Du and also Zihua Zhu from the Pacific Northwest National Laboratory.
Also read

