Researchers develop novel perovskite-type oxide catalysts
- Researchers from Tohoku University as well as Tokyo Institute of Technology (Tokyo Tech) have proposed a simple sol-- gel method for the synthesis of extremely pure bifunctional solid acid − base catalysts of perovskite-type oxides.
The rationale for picking perovskite-type oxides was explained by Professor Keigo Kamata from Tokyo Institute of Technology: "Perovskite-type oxides are acquiring importance in several areas, including magneticity, ferroelectricity, piezoelectricity, as well as catalysis. Moreover, the structure and physiochemical properties of perovskite-type oxides can be tuned by managing their chemical composition."
Perovskite-type oxides have a versatile structure and also demonstrate good architectural stability, flexibility, and also controllability for a number of catalytic reactions. Nonetheless, they have not been completely explored for acid-- base-catalyzed responses. Moreover, the existing methods for synthesis are complex, time-consuming, and call for multiple steps, making it hard to synthesize very pure and desirable perovskite oxide catalysts.
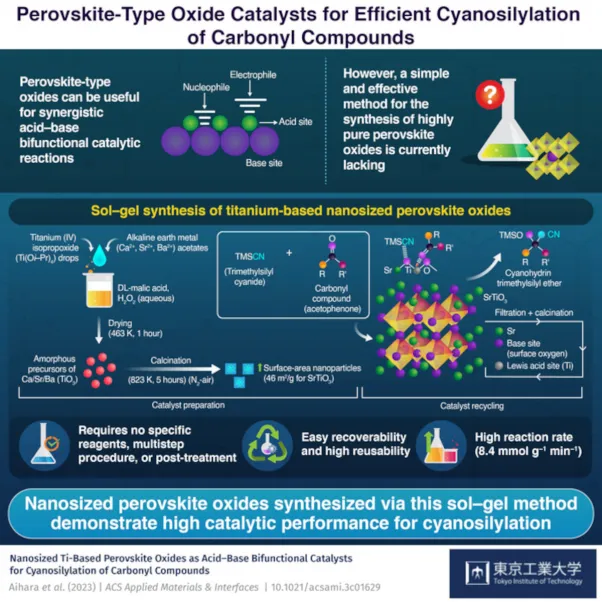
The sol-- gel method developed by the team to circumvent these difficulties has 2 key steps. The initial includes preparing the precursor perovskite material, while the second comprises a calcination procedure to enhance the surface area of the product.
To prepare the precursor, the researchers liquified a metal alkoxide containing either titanium (Ti4+), zirconium (Zr4+), or niobium (Nb5+) cations to an option containing malic acid and also hydrogen peroxide. They then included a steel acetate containing an A-site element to the solution and dried it to form a powdered precursor.
Next off, they subjected the precursor to a calcination procedure by warming it to 550 ° C in a nitrogen atmosphere and also subjecting it to air for 5 hours. The high temperature as well as the presence of oxygen crystallized the precursor and also increased its area.
Of the various catalysts synthesized using this method, the one with SrTiO3 nanoparticles presented the greatest catalytic task. It made it possible for reliable cyanosilylation of acetophenone with trimethylsilyl cyanide (TMSCN) under mild response conditions without any thermal pretreatment.
Upon analyzing the catalyst better, the team found its certain surface area (46 m2/g) to be 10 times higher than those of readily available titanates (1-- 4 m2/g). Prof. Kamata adds, "We observed that the synthesized nanoparticles could trigger TMSCN on the basic oxygen site and also the carbonyl compound on the acidic Ti website. This cooperative activation mechanism was accountable for the high catalytic task of the catalyst for the cyanosilylation reaction."
The team's simple method for synthesizing very pure perovskite oxides for catalysis can come to be a promising technique for making it possible for reactions requiring moderate problems.
Also read

