Liquid-like activity in crystals could describe their encouraging actions in solar cells
- The sun supplies much more energy to Earth in one hour than mankind consumes over a whole year. Researchers worldwide are looking for materials that can cost-effectively and also efficiently record this carbon-free energy and transform it into power.
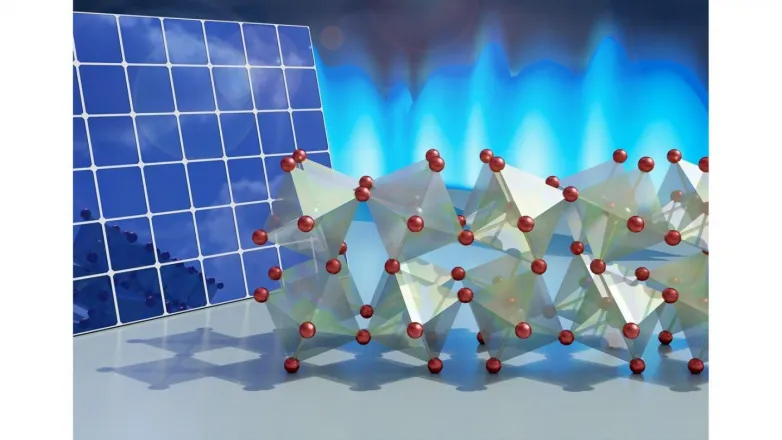
Perovskites, a class of products with an unique crystal structure, could overtake existing modern technology for solar energy harvesting. They are less expensive than materials utilized in current solar cells, and they have actually shown impressive photovoltaic buildings-- actions that enables them to extremely successfully convert sunshine into electrical energy.
Exposing the nature of perovskites at the atomic scale is important to comprehending their encouraging abilities. This understanding can assist educate models to identify the ideal makeup of perovskite products for solar cells, which can be utilized to power cars, digital tools and also even residence heating and also various other devices.
Researchers at the UNITED STATE Division of Energy's (DOE) Argonne National Laboratory joined a cooperation led by Duke University, together with DOE's Oak Ridge National Laboratory and other partners, to examine the inner functions of a perovskite product making use of the world-class X-ray scattering capabilities at Argonne as well as neutron spreading capabilities at Oak Ridge. The spreading capabilities allowed the researchers to observe the product's actions at the atomic scale, and also the research exposed that liquid-like movement in perovskites might describe how they efficiently produce electric currents.
" There is a lot of excitement bordering these materials, yet we don't completely understand why they are such great photovoltaics," said Duke University's Olivier Delaire, lead scientist on the research.
When light hits a solar product, it excites electrons, motivating them to bulge of their atoms as well as travel with the product, conducting electrical energy. A typical problem is that the ecstatic electrons can recombine with the atoms rather than traveling via the material, which can substantially reduce the electrical power created about the amount of sunshine striking the product.
" Perovskites do well at stopping recombination," said Argonne's Ray Osborn. "We need to know what system triggers this and also if we can gain from this to develop much better solar cells."
The group researched among the easiest perovskites-- a substance of cesium, lead and bromine (CsPbBr3)-- to figure out what is taking place at the atomic scale.
Making use of X-ray scattering abilities at Argonne's Magnetic Materials team's beamline (6-ID-D) at the laboratory's Advanced Photon Source, a DOE Workplace of Scientific Research Customer Center, the team captured the typical positions of the atoms in a perovskite crystal at various temperature levels. They found that each lead atom and its surrounding cage of bromine atoms form rigid devices that act like molecules. These devices oscillate-- or jiggle back and forth-- in a liquid-like manner.
"The particles in this product revolve concerning the other particles like they're hinged with each other, as well as around the hinges, the particles act type of saggy," said Delaire.
One theory to explain just how perovskites stand up to recombination is that these distortions in the latticework, or crystal structure, follow the cost-free electrons as they go across the product. The electrons might deform the lattice, creating the liquid-like disturbances, which then prevent them from falling back into their host atoms. This concept, which is bolstered by the new experimental results, can provide brand-new insights into exactly how to develop optimal perovskite products for solar cells.
The data likewise indicates that molecules in the material oscillate within two-dimensional planes, without any activity across airplanes-- similar to a carnival ride that only swings delegated right, yet never front to back. The two-dimensional nature of the crystal distortions could be another puzzle item to describe just how the perovskite can stop electron recombination, adding to the efficiency of the material.
According to Osborn, the two-dimensional patterns in the X-ray scattering information had actually never ever been seen. "Based upon these unforeseen dimensions, we intended to dig even deeper by not only taking a look at typical atomic positions, yet exactly how the atoms move around in live," he claimed.
To examine the activity of the atoms directly, the group used neutron scattering abilities at the Spallation Neutron Resource, a DOE Workplace of Science user center at Oak Ridge National Laboratory. Researchers at Argonne's Products Scientific research department and also Northwestern University expanded the big, centimeter-scale crystals needed for the neutron dimensions.
The neutron spreading validated the unexpected pattern seen in the X-ray scattering experiment, however showed, on top of that, that it takes almost no energy for the particles to oscillate in two dimensions. This helps to discuss why the ecstatic electrons can flaw the latticework so conveniently.
"This work is a lovely example of the complementarity of neutrons and X-rays in revealing both the structure and characteristics of intricate products," stated Osborn, that was involved in both collections of measurements.
The research study stands for an action in the direction of maximizing the largely untapped renewable energy from the sunlight, which could have substantial impact on both the setting as well as economic climate.
Also read

