Hydrogen in crossbreed perovskites is less innocent than it looks
- Researchers in the products department in UC Santa Barbara's College of Engineering have uncovered a significant reason for constraints to effectiveness in a brand-new generation of solar cells.
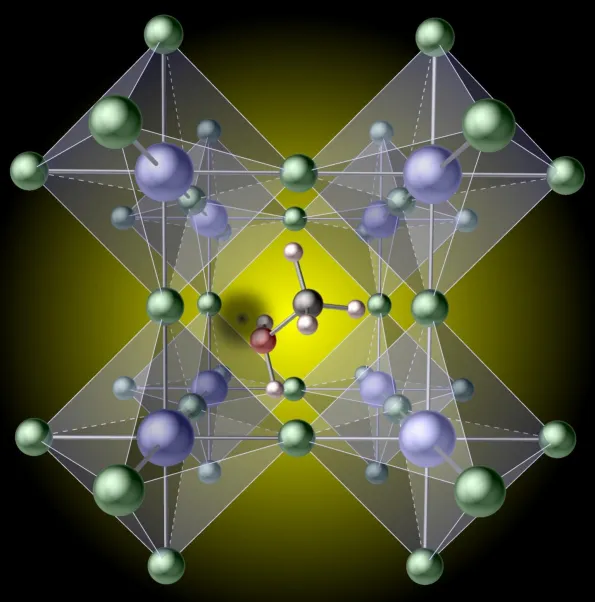
Different feasible flaws in the lattice of what are called hybrid perovskites had actually previously been considered as the prospective source of such constraints, yet it was thought that the organic molecules (the components in charge of the "hybrid" name) would continue to be undamaged. Cutting-edge calculations have currently disclosed that missing hydrogen atoms in these molecules can trigger enormous effectiveness losses. The findings are published in a paper titled "Lessening hydrogen vacancies to enable extremely efficient crossbreed perovskites," in the April 29 concern of the journal Nature Products.
The exceptional photovoltaic efficiency of crossbreed perovskites has actually produced a good deal of enjoyment, offered their prospective to progress solar-cell technology. "Hybrid" refers to the embedding of natural molecules in an inorganic perovskite latticework, which has a crystal framework similar to that of the perovskite mineral (calcium titanium oxide). The materials display power-conversion effectiveness matching that of silicon, but are much cheaper to produce. Problems in the perovskite crystalline lattice, however, are recognized to produce unwanted power dissipation in the form of heat, which restricts performance.
A number of research study groups have been examining such problems, amongst them the team of UCSB materials professor Chris Van de Walle, which just recently attained an advancement by discovering a harmful issue in a place no one had actually looked before: on the organic molecule.
" Methylammonium lead iodide is the normal hybrid perovskite," discussed Xie Zhang, lead researcher on the task. "We found that it is surprisingly easy to break one of the bonds as well as remove a hydrogen atom on the methylammonium molecule. The resulting 'hydrogen job' after that works as a sink for the electrical charges that relocate through the crystal after being produced by light falling on the solar cell. When these charges obtain caught at the job, they can no longer do beneficial work, such as billing a battery or powering a motor, thus the loss in effectiveness."
The study was allowed by innovative computational techniques developed by the Van de Walle group. Such modern computations offer detailed details regarding the quantum-mechanical habits of electrons in the material. Mark Turiansky, a senior graduate student in Van de Walle's group who was associated with the research, aided build innovative approaches for turning this information right into measurable values for rates of charge service provider capturing.
" Our group has actually produced effective methods for establishing which refines reason effectiveness loss," Turiansky said, "and it is gratifying to see the strategy offer such beneficial understandings for an important class of materials."
" The calculations serve as an academic microscopic lense that enables us to peer into the material with a lot higher resolution than can be accomplished experimentally," Van de Walle described. "They likewise create a basis for reasonable materials layout. With experimentation, it has actually been located that perovskites in which the methylammonium molecule is changed by formamidinium exhibit much better performance. We are currently able to attribute this renovation to the truth that hydrogen flaws develop less readily in the formamidinium compound.
" This insight supplies a clear reasoning for the empirically established knowledge that formamidinium is crucial for realizing high-efficiency solar cells," he included. "Based on these fundamental understandings, the researchers who make the products can develop methods to suppress the hazardous flaws, increasing additional effectiveness improvements in solar cells."
Also read

