Zinc-air battery with enhanced efficiency thanks to solar power
- Zinc-air batteries, which generate electricity with a chemical reaction in between oxygen in the atmosphere as well as zinc, are considered to be next-generation prospects to meet the explosive demand for electric vehicles as opposed to lithium-ion batteries.
They in theory meet all called for characteristics for next-generation secondary batteries, such as high energy thickness, low risk of surge, eco-friendliness (does not emit toxins), and also inexpensive of materials (zinc as well as air, which can be easily obtained from nature).
The Korea Institute of Science and also Technology (KIST) introduced that its study team led by Dr. Joong Kee Lee (Energy Storage Research Center) established a technology to enhance the electrochemical performance of zinc-air batteries by using solar energy, which is becoming a new research and development location in the secondary battery area.
The battery established by the study group utilizes a photoactive bifunctional air-electrocatalyst with a semiconductor structure with rotating energy levels, which significantly improves the rates of oxygen reduction reaction (ORR) and also oxygen evolution reaction (OER) that produce electricity. The photoactive bifunctional catalyst is a substance that accelerates chain reactions by taking in light energy and has an improved light absorption ability than conventional zinc-air battery catalysts.
In a zinc-air battery that makes use of steel and air as the anode and cathode of the battery, OER and also ORR need to be at the same time carried out for electrical energy conversion of oxygen as the cathode active material. As a result, the catalytic activity of the positive electrode current collector, constructed from carbon material, is a crucial factor in figuring out the energy thickness and total cell effectiveness of zinc-air batteries.
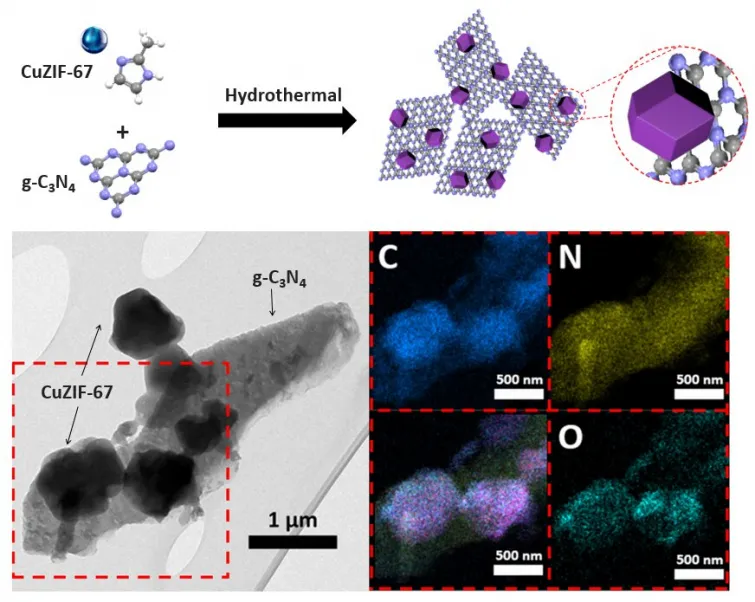
Appropriately, the KIST research study team focused on the p-n heterojunction, the fundamental structural system of solar cells as well as semiconductors, as a procedure to enhance the slow catalytic activity of zinc-air batteries. The goal was to accelerate the oxygen production-reduction process by utilizing the user interface characteristics of semiconductors in which electron movement occurs. To this end, a cathode material with a heterojunction bandgap framework was synthesized, with a n-type semiconductor (graphitic carbon nitride, g-C3N4) and ap-type semiconductor (copper-doppedZIF-67( ZeoliticImidazolateFramework-67), CuZIF-67).
On top of that, an experiment was carried out under real-world problems without light in order to confirm the commercial capacity of the photoactive bifunctional catalyst with a p-n heterojunction structure with alternating energy levels. The prototype battery revealed an energy density of 731.9 mAh gZn-1, comparable to the most effective performance of the existing zinc-air battery. In the existence of sunlight, the energy density boosted by regarding 7% as much as 781.7 mAh gZn-1 and exceptional cycle efficiency (334 hours, 1,000 cycles), displaying the very best efficiency among known catalysts.
Dr. Lee says that "use of solar energy is an important part not only in boosting the electrochemical performance of secondary batteries yet additionally in realizing a lasting society. We hope that this technology will end up being a catalyst that stimulates the development of brand-new merging technologies in semiconductor physics as well as electrochemistry, along with solving the difficulties of metal-air batteries that are becoming an alternative to lithium-ion batteries."
The research study was released in Applied Catalysis B: Environmental.
Also read

