Scientists catch honest photos of electrons collecting light at the atomic scale
- In the look for tidy energy options to nonrenewable fuel sources, one appealing remedy depends on photoelectrochemical (PEC) cells - water-splitting, artificial-photosynthesis devices that transform sunshine and also water into solar fuels such as hydrogen.
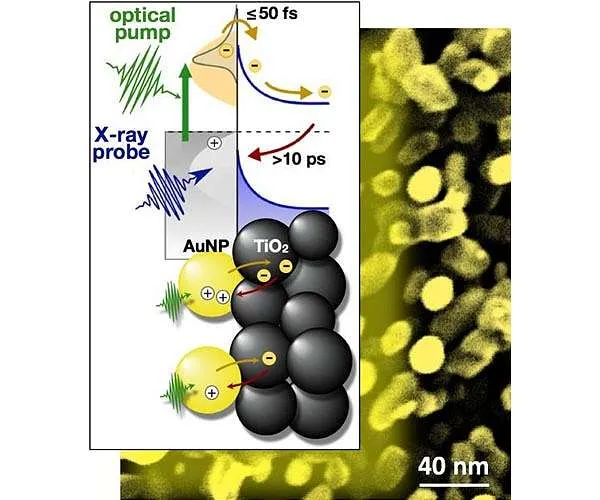
In simply a years, scientists in the field have attained great development in the growth of PEC systems made from light-absorbing gold nanoparticles - small rounds just billionths of a meter in size - affixed to a semiconductor film of titanium dioxide nanoparticles (TiO2 NP). However regardless of these advancements, researchers still struggle to make a gadget that can generate solar fuels on an industrial scale.
Now, a team of researchers led by the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) has actually gotten essential brand-new understanding right into electrons' duty in the harvesting of light in gold/TiO2 NP PEC systems. The researchers claim that their research, recently published in the Journal of Physical Chemistry Letters, can help researchers develop extra efficient material combinations for the layout of high-performance solar fuels gadgets.
" By measuring just how electrons do their work with the nanoscale and also in real time, our research study can aid to discuss why some water-splitting PEC tools did not work as well as hoped," claimed elderly author Oliver Gessner, an elderly scientist in Berkeley Lab's Chemical Sciences Division.
And also by tracing the motion of electrons in these complicated systems with chemical specificity as well as picosecond (trillionths of a second) time resolution, the study team members believe they have actually developed a new tool that can a lot more accurately calculate the solar fuels conversion efficiency of future gadgets.
Electron-hole pairs: An effective pairing comes to light
Researchers studying water-splitting PEC systems have had an interest in gold nanoparticles' superior light absorption due to their "plasmonic vibration" - the capacity of electrons in gold nanoparticles to relocate sync with the electric field of sunlight.
" The method is to transfer electrons in between two different kinds of products - from the light-absorbing gold nanoparticles to the titanium-dioxide semiconductor," Gessner clarified.
When electrons are moved from the gold nanoparticles into the titanium dioxide semiconductor, they leave behind "openings." The combination of an electron injected right into titanium dioxide and also the hole the electron left is called an electron-hole pair. "And we know that electron-hole pairs are important components to allowing the chain reaction for the production of solar fuels," he included.
Yet if you need to know how well a plasmonic PEC gadget is functioning, you need to discover the amount of electrons moved from the gold nanoparticles to the semiconductor, the number of electron-hole sets are created, and for how long these electron-hole sets last prior to the electron returns to a hole in the gold nanoparticle.
" The longer the electrons are divided from the holes in the gold nanoparticles - that is, the longer the lifetime of the electron-hole sets - the more time you have for the chemical reaction for fuels production to happen," Gessner discussed.
To answer these concerns, Gessner and his group used a technique called "picosecond time-resolved X-ray photoelectron spectroscopy (TRXPS)" at Berkeley Lab's Advanced Light Source (ALS) to count how many electrons transfer in between the gold nanoparticles as well as the titanium-dioxide film, and also to measure for how long the electrons remain in the other product.
Gessner stated his team is the very first to use the X-ray strategy for studying this transfer of electrons in plasmonic systems such as the nanoparticles and the film. "This information is essential to establish extra reliable material combinations."
An electronic 'count'- down with TRXPS
Using TRXPS at the ALS, the team shone pulses of laser light to thrill electrons in 20-nanometer (20 billionths of a meter) gold nanoparticles (AuNP) connected to a semiconducting film constructed from nanoporous titanium dioxide (TiO2).
The team then made use of brief X-ray pulses to measure how many of these electrons "took a trip" from the AuNP to the TiO2 to create electron-hole sets, and then back "home" to the holes in the AuNP.
" When you want to take an image of somebody relocating really quick, you do it with a brief flash of light - for our research study, we utilized short flashes of X-ray light," Gessner stated. "And our camera is the photoelectron spectrometer that takes short 'snapshots' at a time resolution of 70 picoseconds."
The TRXPS dimension exposed a few surprises: They observed 2 electrons transfer from gold to titanium dioxide - a far smaller sized number than they had actually expected based on previous research studies. They also discovered that only one in 1,000 photons (fragments of light) created an electron-hole pair, which it takes just a billionth of a second for an electron to recombine with an opening in the gold nanoparticle.
Altogether, these searchings for as well as approaches defined in the existing research might aid scientists much better approximate the optimum time required to cause solar fuels manufacturing at the nanoscale.
" Although X-ray photoelectron spectroscopy is a common method made use of at universities and also research organizations all over the world, the method we increased it for time-resolved research studies as well as utilized it below is very unique and also can only be done at Berkeley Lab's Advanced Light Source," claimed Monika Blum, a co-author of the research as well as study researcher at the ALS.
" Monika's as well as Oliver's distinct use TRXPS made it feasible to recognize the amount of electrons on gold are activated to become cost service providers - and also to find and also track their activity throughout the surface area of a nanomaterial - with unmatched chemical specificity and picosecond time resolution," stated co-author Francesca Toma, a team researcher at the Joint Center for Artificial Photosynthesis (JCAP) in Berkeley Lab's Chemical Sciences Division. "These searchings for will be essential to obtaining a far better understanding of just how plasmonic products can progress solar fuels."
The team next strategies to press their measurements to even faster time scales with a free-electron laser, and also to record even better nanoscale pictures of electrons at the office in a PEC gadget when water is added to the mix.
Also read
- CNNP Optoelectronics brings utility-scale perovskite modules out of the lab
- Low-Temperature Sequential Deposition Lifts Inverted Perovskite Solar Cells Efficiency Record
- Self-Assembling Molecule Breakthrough Brings Commercial Perovskite Solar Closer to Market
- Camphor Additives Boost Perovskite Solar Cell Efficiency
- NUS Sets Record With 26.4% Perovskite-Organic Solar Cell
