Researchers uncover how oxygen loss saps a lithium-ion battery's voltage
- When lithium ions flow in and out of a battery electrode throughout billing as well as releasing, a little bit of oxygen permeates out and the battery's voltage-- a procedure of just how much energy it supplies-- discolors an equally little bit. The losses place with time, as well as can ultimately sap the battery's energy storage capacity by 10-15%.
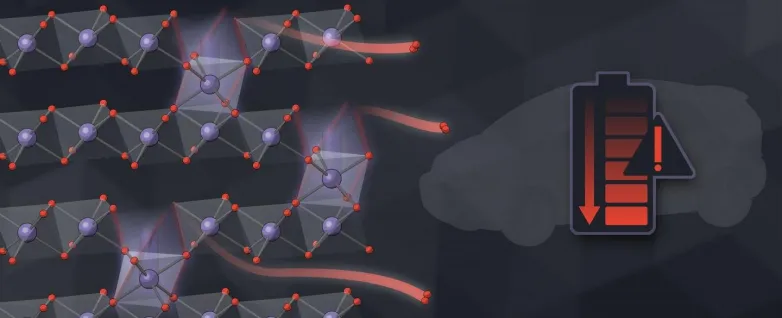
Now scientists have actually gauged this super-slow procedure with unprecedented information, showing how the holes, or jobs, left by running away oxygen atoms transform the electrode's framework as well as chemistry and also gradually decrease how much energy it can keep.
The results negate several of the presumptions scientists had actually made about this process as well as can suggest brand-new methods of engineering electrodes to stop it.
The research team from the Department of Energy's SLAC National Accelerator Laboratory and Stanford College defined their work in Nature Energy today.
" We had the ability to determine a very little degree of oxygen trickling out, ever so gradually, over thousands of cycles," claimed Peter Csernica, a Stanford Ph.D. student that worked with the experiments with Associate Professor Will Chueh. "The reality that it's so slow is additionally what made it hard to find."
A two-way shaking chair
Lithium-ion batteries function like a rocking chair, relocating lithium ions back and forth between 2 electrodes that temporarily store cost. Ideally, those ions are the only things moving in and out of the billions of nanoparticles that comprise each electrode. However researchers have known for a long time that oxygen atoms leakage out of the particles as lithium moves back and forth. The information have been difficult to determine because the signals from these leaks are also small to determine directly.
" The total amount of oxygen leak, over 500 cycles of battery charging as well as discharging, is 6%," Csernica claimed. "That's not such a small number, yet if you try to gauge the quantity of oxygen that appears throughout each cycle, it has to do with one one-hundredth of a percent."
In this research study, scientists gauged the leak indirectly instead, by considering how oxygen loss modifies the chemistry as well as framework of the particles. They tracked the process at a number of length scales-- from the tiniest nanoparticles to clumps of nanoparticles to the full thickness of an electrode.
Because it's so hard for oxygen atoms to move around in solid materials at the temperatures where batteries run, the conventional wisdom has been that the oxygen leaks come just from the surface areas of nanoparticles, Chueh said, although this has been up for argument.
To obtain a more detailed look at what's occurring, the study group cycled batteries for various amounts of time, took them apart, and also cut the electrode nanoparticles for detailed assessment at Lawrence Berkeley National Laboratory's Advanced Light Source. There, a specialized X-ray microscope checked across the samples, making high-res pictures as well as probing the chemical make-up of each small spot. This info was combined with a computational method called ptychography to reveal nanoscale information, determined in billionths of a meter.
At the same time, at SLAC's Stanford Synchrotron Source of light, the team shot X-rays via entire electrodes to validate that what they were seeing at the nanoscale degree was also real at a much larger scale.
A burst, after that a trickle
Comparing the experimental outcomes with computer system models of how oxygen loss might occur, the team ended that an initial ruptured of oxygen escapes from the surfaces of particles, followed by a very slow-moving trickle from the interior. Where nanoparticles glommed with each other to develop bigger globs, those near the center of the glob shed much less oxygen than those near the surface.
An additional essential inquiry, Chueh said, is exactly how the loss of oxygen atoms impacts the material they left behind. "That's in fact a large secret," he claimed. "Envision the atoms in the nanoparticles are like close-packed rounds. If you maintain taking oxygen atoms out, the entire thing can crash down as well as densify, because the framework likes to remain closely stuffed."
Considering that this aspect of the electrode's structure can not be straight imaged, the scientists once more compared other kinds of experimental monitorings against computer system models of various oxygen loss circumstances. The outcomes showed that the vacancies do persist-- the material does not crash down and densify-- as well as suggest how they contribute to the battery's progressive decline.
" When oxygen leaves, surrounding manganese, nickel and cobalt atoms migrate. All the atoms are dancing out of their suitable positions," Chueh stated. "This rearrangement of metal ions, in addition to chemical modifications caused by the missing out on oxygen, breaks down the voltage and performance of the battery gradually. Individuals have understood aspects of this sensation for a long time, but the device was unclear."
Currently, he claimed, "we have this clinical, bottom-up understanding" of this crucial resource of battery deterioration, which could bring about new means of mitigating oxygen loss and its destructive impacts.
Also read

