Protecting against dye aggregation with molten salts to improve solar cell performance
- Solar cells are swiftly turning into one of the major ways to create clean electrical energy in lots of nations in the world. Over the past few years, a tremendous amount of initiative has been committed to making solar energy extra prominent. However, the technology currently faces several difficulties that limit widespread application.
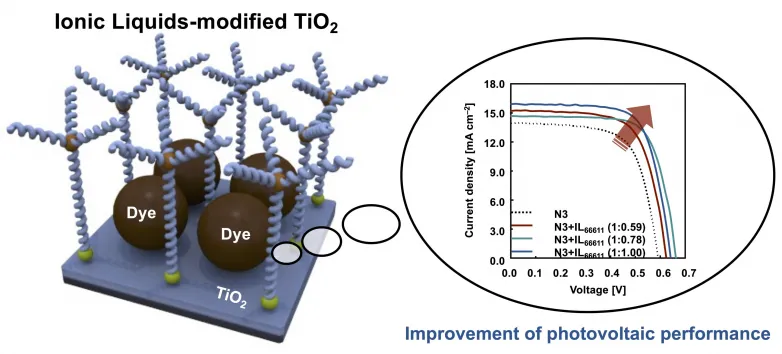
When it comes to dye-sensitized solar cells (DSSCs)-- an extremely promising photovoltaic or pv technology-- one of the main problems is dye aggregation. Deliberately, DSSCs are electrochemical systems that mimic photosynthesis in plants; they rely upon unique photosensitive dyes to convert sunlight right into electrical energy. Preferably, the dye must be applied equally over the surface area of an oxide electrode behind a clear layer so that energy from absorbed sunlight can be transferred quickly to the dye's electrons. This process generates cost-free electrons that power an external circuit. However, many dyes have a tendency to aggregate over the electrode surface in a manner that hinders the wanted flow of both light as well as electric fees. This takes a toll on the performance of DSSCs that has proven hard to overcome.
Fortunately, a team of scientists led by Associate Professor Tomohiko Inomata of Nagoya Institute of Technology, Japan, might have just found a remedy to this problem. In their recent research study released in RSC Advances, they revealed that certain ionic liquids (molten salts that are in liquid state at fairly low temperatures) can suppress dye gathering to an excellent level. Various other members of this research group consisted of Ms. Ayaka Matsunaga and Prof. Tomohiro Ozawa from Nagoya Institute of Technology, and also Prof. Hideki Masuda from Aichi Institute of Technology, Japan.
Yet, exactly how do ionic liquids accomplish this feat? To shed light on the precise system at play, the scientists focused on 2 ionic liquids with substantially various molecular sizes and also 2 sorts of dyes. Both the ionic liquids had a comparable molecular framework making up an anchor that binds well to the electrode (titanium dioxide, TiO2), a major polymer chain linking this anchor to a phosphor atom, and also 3 extra short polymer chains sticking out from the phosphor atom and far from the major "vertical" chain.
The scientists immersed the TiO2 electrodes in solutions with different dye-to-ionic-liquid percentages and very carefully examined just how the different molecules adhered to them. After optimizing the synthesis treatment, they found that DSSCs used the ionic liquid with a longer molecular structure had an incredibly far better performance than their counterparts with non-modified oxide electrodes. "The spatially bulky molecular framework of ionic liquids functions as an effective anti-aggregation agent without substantially affecting the quantity of dye adsorbed right into the electrode," discusses Dr. Inomata. "Most significantly, the intro of the bigger ionic liquid improves all the photovoltaic or pv parameters of the DSSCs."
Needless to say, improving solar cell technology can give us an edge in the fight versus the ongoing energy and also climate situation. Although ionic liquids are normally costly, the way it is utilized by the group is, as a matter of fact, cost-efficient. "Simply put, the idea is to apply ionic liquids only at the needed part of the gadget-- in this case, the electrode's surface area," specifies Dr. Inomata.
The group believes that the widespread use electrodes changed with ionic liquids could pave the way for extremely practical yet affordable products for solar cells as well as catalytic systems. Considering that the framework of ionic liquids can be tuned during their synthesis, they offer a much-needed versatility as anti-aggregation representatives.
Also read

