Nonflammable electrolyte for high-performance potassium batteries
- Australian scientists have actually developed a nonflammable electrolyte for potassium and potassium-ion batteries, for applications in next-generation energy-storage systems beyond lithium technology.
In the journal Angewandte Chemie, researchers compose that the novel electrolyte based upon an organic phosphate makes the batteries safer and also enables operation at decreased concentrations, which is a necessary condition for large-scale applications.
Lithium-ion innovation still dominates energy-storage applications, however it has intrinsic drawbacks, among which are the cost, environmental concerns, and the flammability of the electrolyte.
Therefore, in next-generation innovations, scientists are replacing the lithium ion with more plentiful and much cheaper ions, such as the potassium ion. However, potassium and potassium-ion batteries also deal with safety issues, and nonflammable electrolytes are not yet readily available for them.
Materials researcher Zaiping Guo, and her group from the University of Wollongong, Australia have found an option. The scientists developed an electrolyte based on a flame-retardant material and adjusted it for usage in potassium batteries. Besides providing nonflammability, it could be run in batteries at concentrations that appropriate for massive applications, compose the researchers.
This unique electrolyte included triethyl phosphate as the sole part of the solvent. This compound is called a flame retardant. It has been checked in lithium-ion batteries, but only very high concentrations supplied enough stability for long-lasting operation, too high for commercial applications.
The battery industry demands water down electrolytes, which are more affordable and guarantee much better performances. By utilizing potassium ions, nevertheless, the concentrations might be reduced, the authors reported.
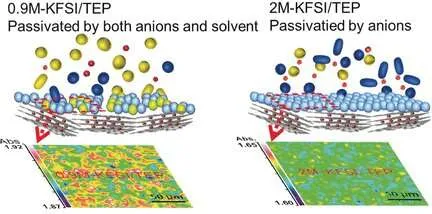
They integrated the phosphate solvent with a frequently readily available potassium salt and obtained an electrolyte that did not burn and allowed stable biking of the put together battery concentrations of 0.9 to 2 moles per liter, which are concentrations that are appropriate for bigger scales; for instance, in smart-grid applications.
Key to that efficiency was the formation of a uniform and steady solid-electrolyte interphase layer, according to the authors. They observed this layer, which makes sure operability of the electrodes, only with the phosphate electrolyte.
Conventional carbonate-based electrolytes were not able to develop up this layer. The authors likewise reported high cycling stability; whereas, under the very same conditions, the traditional carbonate-based electrolyte decayed.
Guo and her group have shown that next-generation potassium-ion batteries can be made safe by utilizing an unique inorganic, phosphate-based electrolyte. They recommend that electrolytes based on flame retardants can be established further and might be used for the design of other nonflammable battery systems.
Also read

