A much more efficient means to discover a much more reliable battery
- The rate of development in the renewable energy sector is restricted not only by the technology to catch energy from the sun, the wind, the seas or the Earth's convected heat, however additionally by the capacity to properly keep and also deploy that energy after it has been utilized.
The key hurdle in the advancement of reliable batteries that can be scaled to offer the requirements of the grid is, unsurprisingly, expense-- of products, yet additionally of research necessary to locate the very best products.
At Washington University in St. Louis, a research group in the laboratory of Vijay Ramani, the Roma B. & Raymond H. Wittcoff Distinguished University Professor at the McKelvey School of Engineering, has developed a method to establish which products will certainly be suitable as a vital component in any type of organic redox flow battery (ORFB) for grid-scale energy storage space: the electrolyte.
The study was released Aug. 20 in the journal Process of the National Academy of Sciences (PNAS).
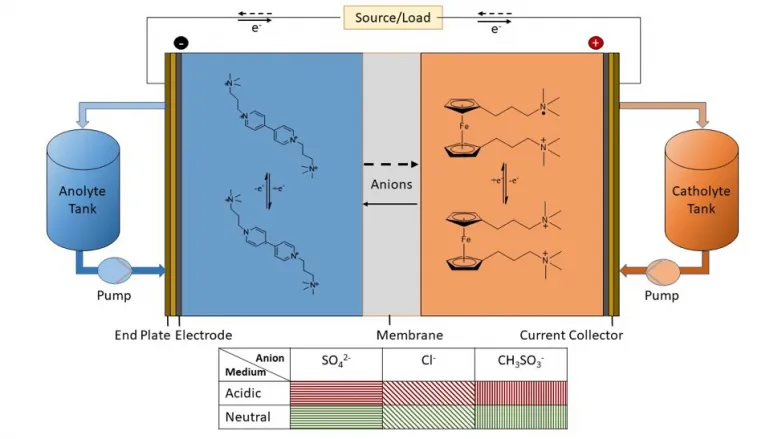
Organic redox flow batteries (ORFB) are affordable. Their design makes them more affordable than lithium-ion batteries per unit of electrical energy stored at range, as well as they utilize affordable natural materials for the battery actives (cathode and also anode).
" In our system, we are using viologen, which is commonly used as a herbicide as well as is very low-cost," claimed very first author Kritika Sharma, a Ph.D. trainee in Ramani's laboratory. "If we are utilizing such natural actives, then the primary decision is, 'What electrolyte do we liquify it in to make best use of battery efficiency?'" she stated.
Typically, responding to that question has actually included a great deal of experimental experimentation and analyses. What Ramani's group located, nonetheless, has the potential to remove much of this work: An universal descriptor that shows which electrolytes are best coupled with natural actives.
Ramani's research study group, consisting of Shrihari Sankarasubramanian and Javier Parrondo, in addition to Sharma, considered 2 actives (a ferrocene dichloride cathode as well as a propyl viologen tetrachloride anode) and also six electrolytes (sulfuric acid; hydrochloric acid; methane sulfonic acid; sodium sulfate; sodium chloride; as well as sodium methane sulfonate) at neutral as well as acidic pH. They located their universal descriptor suggested the combinations with one of the most complementary chemistry as well as battery performance attributes-- low discharge polarization as well as high open circuit voltage.
" Our descriptor i.e., solvent reorganizational energy, enabled us to reveal that low pH electrolytes with methane sulfonate or chloride counterions worked best," claimed Sankarasubramanian, joint very first author on the paper and also assistant professor of chemical engineering at the University of Texas at San Antonio. "We were able to anticipate this with an hour of trial and error in the lab rather than the usual days or weeks."
Although the paper reveals results from a limited number of combinations, Sharma said the descriptor can be generalized as it is based upon the basic relationship in between the actives and electrolytes and associates the kinetic and also transport properties in the system.
With a generalizable approach to anticipate the very best electrolytes for a provided natural energetic, the development of new storage modern technologies will certainly become much more effective-- as well as none ahead of time.
" Grid-scale power storage space is called for to have a steady grid when recurring solar- and wind-based generators are dominant," Sharma claimed. "Our global descriptor can assist accelerate the development of brand-new storage space services."
Also read

