A make over at the issue of energy effectiveness in lithium-ion batteries
- A worldwide research team including two Skoltech researchers has actually experimentally demonstrated that a long-lasting description for reduced energy effectiveness in lithium-ion batteries does not hold.
The researchers clarified the phenomenon in terms of sluggish electron transfer between oxygen and shift steel atoms in the cathode, instead of the atoms themselves going through migration. The research appeared Thursday in the journal Nature Chemistry.
The lithium-ion batteries utilized in electric vehicles and gizmos today have concerning half the capability their cousins with lithium-enriched oxide cathodes could deliver. The issue with the last technology is it has reduced performance: You have to spend considerably more power to charge up the battery than it will inevitably give. With time, and particularly for applications consuming much energy, this lost power actually adds up, making that kind of batteries readily unrealistic already.
To unlock the possibility of the batteries with lithium-enriched oxide cathodes, researchers need to comprehend the mechanism behind their inefficiency and specifically where the lost energy goes. The current research study in Nature Chemistry gives speculative proof refuting the formerly held description of the sensation-- practically referred to as voltage hysteresis-- and offers a brand-new theory to make up it.
As a lithium-ion battery obtains billed, lithium ions travel between its two electrodes. Migrating towards the anode, they leave behind jobs in the cathode. The other half of the cycle involves lithium ions going back as the energy obtains expended, claim to power a phone.
" In the meantime, however, some of the shift metal atoms making up the cathode might have momentarily attacked the vacancies and afterwards drew back once more, investing valuable energy on this jumping around. Or two the old theory of voltage hysteresis went," study co-author and Skoltech Ph.D. pupil Anatoly Morozov stated.
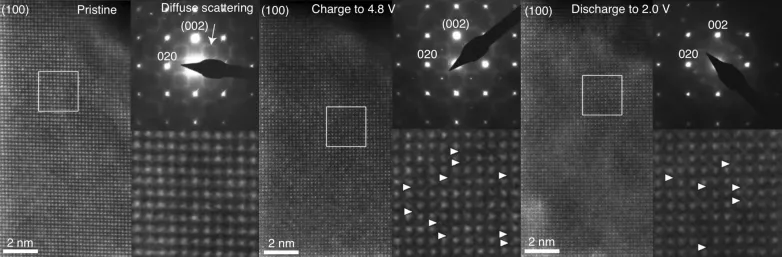
To examine this explanation, the scientists utilized a transmission electron microscopic lense at Skoltech's Advanced Imaging Core Facility to keep track of the atomic framework of a lithium-enriched battery cathode made from a product with the formula Li1.17 Ti0.33 Fe0.5 O2 at different phases in the battery's charge-discharge cycle (see the photo listed below). Nevertheless, no significant movement of iron or titanium atoms to lithium openings was observed, recommending that a few other process was siphoning power.
" Our findings influenced the team to look for the beginning of voltage hysteresis in other places. What generates the sensation is not reversible cation movement yet instead the reversible transfer of electrons in between the atoms of oxygen and transition metals. As the battery gets billed, several of the electrons from iron are pirated by the oxygen atoms. Later, they return. This relatively easy to fix transfer eats a few of the energy," explained Professor Artem Abakumov, who heads the Center of Energy Science and Technology at Skoltech.
" Comprehending voltage hysteresis in terms of electron transfer could have immediate implications for reducing this unwelcome effect to enable next-generation lithium-ion batteries with record-high energy density for powering electrical vehicles and mobile electronic devices," he took place. "To allow that following step, chemists might control the electron transfer obstacles by differing the covalency of the cation-anion bonding, directed by the table of elements and such principles as 'chemical gentleness.'".
" This demonstrates the power of sophisticated transmission electron microscopy for analyzing neighborhood structures of severe complexity. It is truly great that young researchers at Skoltech have direct and very easy access to such sophisticated equipment as aberration-corrected electron microscopic lens, and chances for more training. This allows us to add to top-level battery research in collaboration with our international peers in both academic community and the industry," Morozov added.
Also read

